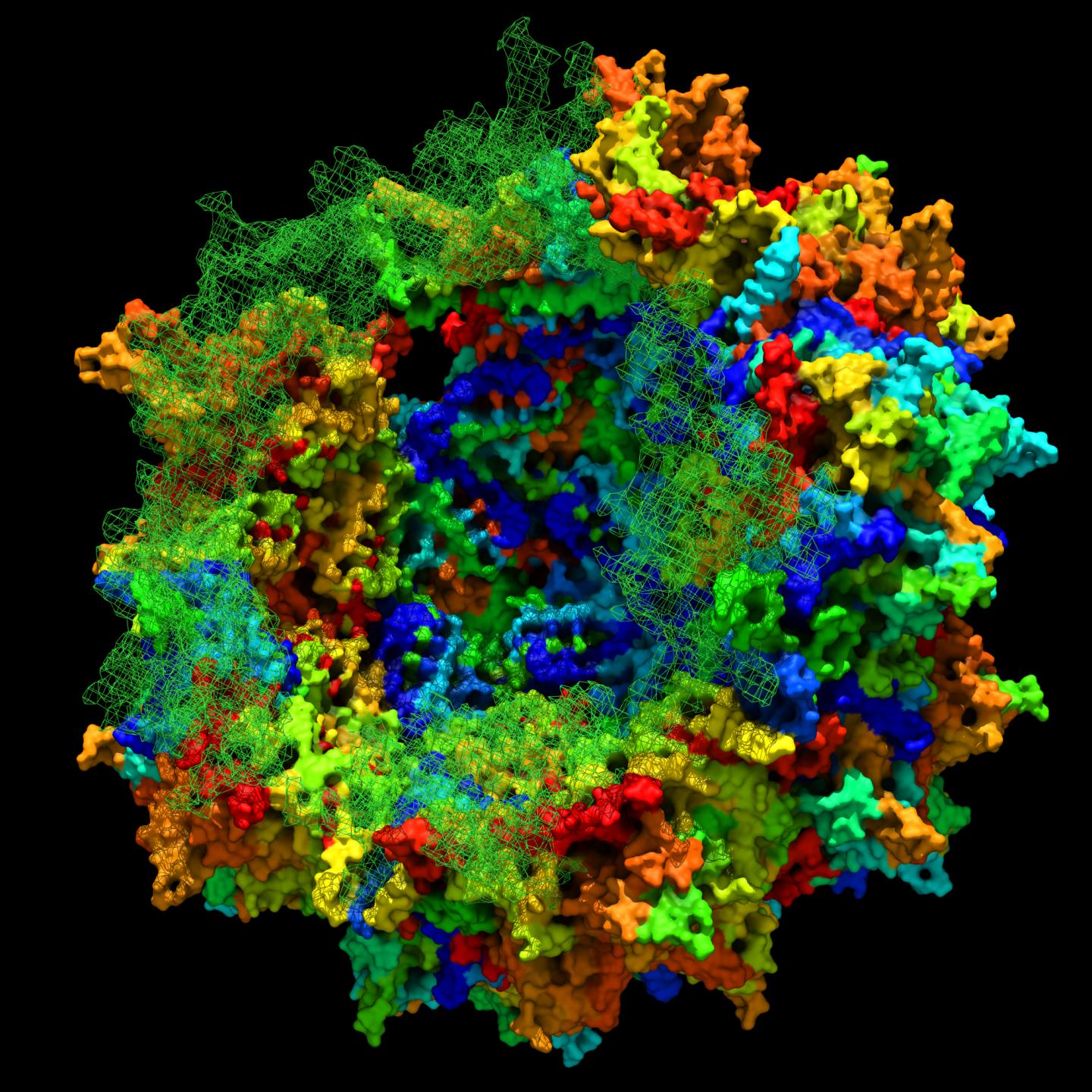
Researchers at the Massachusetts Eye and Ear/Schepens Eye Research Institute have reconstructed an ancient virus they claim is “highly effective at delivering gene therapies to the liver, muscle, and retina”.
The discovery, published on 30 July in Cell Reports, could potentially be used to design gene therapies that are not only safer and more potent than therapies currently available, but may also help a greater number of patients.
“We believe our findings will teach us how complex biological structures, such as AAVs (adeno-associated viruses), are built,” said senior study author Luk H. Vandenberghe, of Mass. Eye and Ear and Harvard Medical School Department of Ophthalmology in a statement on 31 July.
Given its basic nature, a virus can be an ideal delivery system for gene therapy. In order to survive, a virus must infiltrate a host organism undetected and transfer its genetic material into the host’s cells, where it will use the host to replicate and proliferate.
Researchers use this ability of a virus to insert therapeutic genes into it and then use the virus to shuttle the genes to the appropriate cells or tissues inside a human body.
Till date, AAVs used for gene therapy have been chosen from viruses that naturally circulate throughout the human population. If patients have been exposed to the virus, their bodies will likely recognize the virus, mount an attack, and destroy it before it can deliver the therapy.
Engineering new, benign viruses could render the viruses unrecognizable and increase the number of people for whom a given gene therapy will work. However, efforts to engineer improved AAVs have been stymied by the intricate structure of these viruses. Like pieces of a jigsaw puzzle, every protein in the shell of the virus must fit together perfectly for the virus to function normally.
Altering proteins in one part of the virus to achieve a certain benefit, such as more efficient gene transfer or reduced recognition by host immune cells, could end up destroying the structural integrity of the entire shell.
To overcome this challenge, Vandenberghe and his colleagues at Harvard Medical School, Schepens Eye Research Institute, and Mass. Eye and Ear have turned to evolutionary history for guidance.